News
FOR IMMEDIATE RELEASE
RAIsonance Announces Christine Hrudka as Strategic Advisory Lead
for the Pharmacy Industry
Denver Tech Center, Colorado, Jan 10, 2026 — RAIsonance, Inc. today announced the appointment of Ms. Christine Hrudka BSP, ICD.D to the Raisonance Strategic Advisory Board as the Pharmacy Industry Lead. Ms. Hrudka will lead Raisonance’s programs across the digital and in-store pharmacy industry in markets all over the world
Ms. Hrudka brings more than three decades of experience in the pharmacy industry to this appointment, serving most recently as the Chair of the Canadian Pharmacists Association. An accomplished entrepreneur and advocate for women in business, Christine spent 20 years owning and operating Shoppers Drug Mart locations across Saskatchewan before opening independent pharmacies to broaden access to patient-centered care. She has served as the North American representative on the World Pharmacy Council and as a director with Avricore Health, Rapid Dose Therapeutics, and Deepcorp. Christine has also shared her expertise with organizations such as Pharmapod, Aither Ingredient Corp, and Smart Employee Benefits.
A firm believer in the transformative role of pharmacy, Christine is dedicated to using technology to improve patient care and health outcomes. Pharmacies are among the most accessible touchpoints for healthcare, and Christine recognizes the importance of pharmacists building genuine relationships with their patients. With a focus on leveraging innovative solutions, Christine aims to expand access to high-quality care in both urban and rural communities. “I’m truly excited to collaborate with Kitty and the Raisonance team to bring this technology to both digital and brick-and-mortar pharmacies worldwide, making a meaningful difference for patients across the globe,” said Ms. Hrudka.
“I am absolutely thrilled to be working with Christine. With her extensive knowledge of the pharmacy business, Christine will guide the development and strategic implementation of our pharmacy programs to bring meaningful value to both our pharmacy partners and their customers,” said Kitty Kolding, CEO of RAIsonance. “Pharmacies play an integral, familiar, highly accessible role in our healthcare. Our solutions bring convenient, noninvasive, accessible respiratory care to people everywhere. The combination is unbeatable.”
In her new role, Ms. Hrudka will lead Raisonance respiratory disease screening and monitoring programs with the digital and in-store pharmacy industry all over the world. With an initial emphasis on Canada, the United Kingdom, Australia and the US, Ms Hrudka will engage with stakeholders across category, to ensure that Raisonance offerings are available when and where needed for customers, while delivering revenue and services expansion to pharmacy partners.
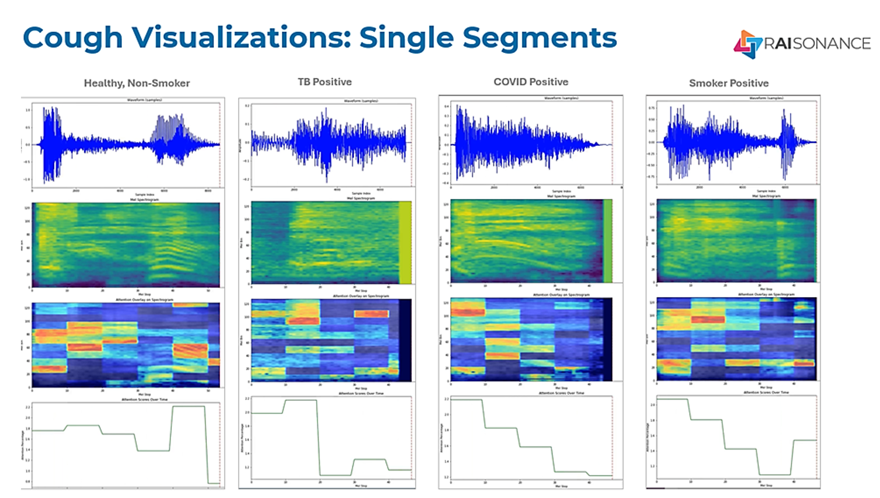
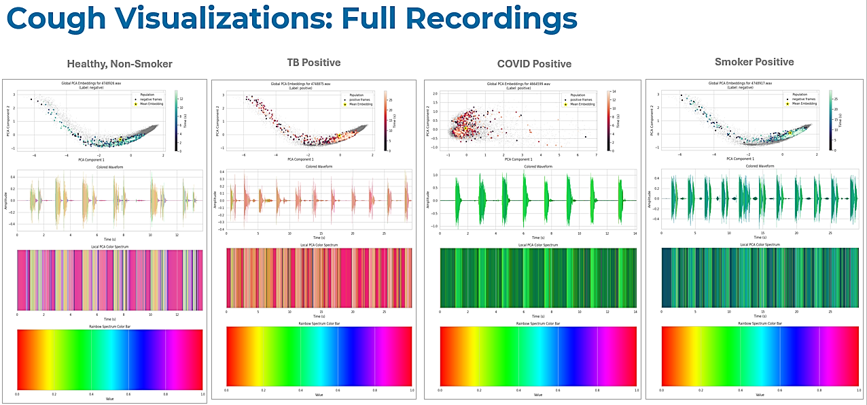
RAIsonance is the world leader in using highly advanced artificial intelligence to analyze and interpret the incredibly rich set of data available from human cough sounds. Their patented, AI-powered solutions analyze more than 5000 individual data points from each cough to identify specific disease signatures and to monitor and score anomalies found in individual users’ respiratory profiles.
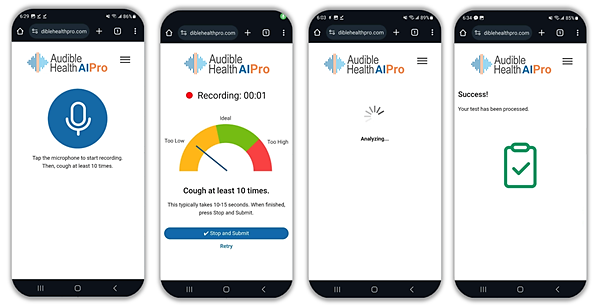
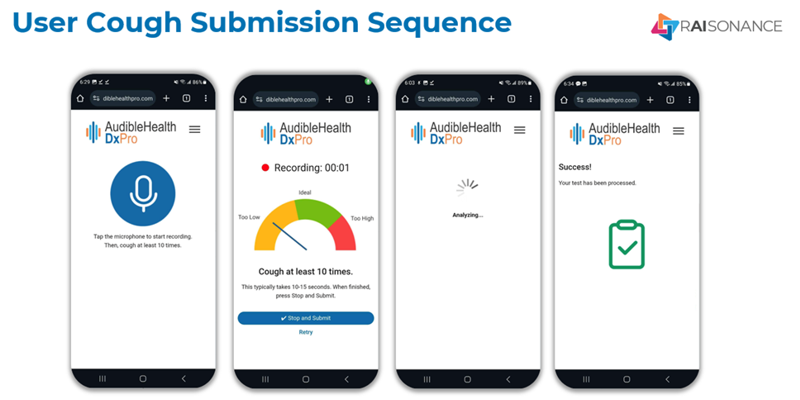
To use Raisonance solutions, an individual coughs into their web app on virtually any Android or Apple smart phone for 10 seconds. That recording is then pushed to the company’s cloud-based production environment in Microsoft Azure, with results returned in approximately 30 seconds. The scalable Azure infrastructure can currently process over 20,000 screenings per second. At present their respiratory disease screening solution, called AudibleHealth AI, can screen for Tuberculosis, COVID-19, Pneumonia, and Influenza, with another 10 test types in active development. The solution is live in 7 countries including the US, UK, and Canada.
The AudibleHealth AI solution is designed to give providers and practitioners a fast, portable, inexpensive, non-invasive way to assess their patients. It works with virtually any Android or Apple smartphone, requires no special equipment or supplies, generates no waste, requires no shipping or warehousing, and works via SMS text message or WhatsApp all over the world. Because the solution works within a mobile browser, no app downloads or software maintenance is required. As a screener, AudibleHealth AI gives providers fast, critical insights to inform care decisions, so they can proceed with appropriate confirmatory testing as needed.
“Our technology has been in development and rigorous testing for over five years and has performed beautifully in all five of our clinical studies. This particular study was our most ambitious yet, and was designed to be an especially challenging test of the overall accuracy and disease distinguishability of our solution – that’s another reason why we’re so thrilled with these results,” said Kitty Kolding, Co-Founder and CEO at RAIsonance.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning-based respiratory healthcare solutions. RAIsonance companies operate a suite of products to detect respiratory illnesses and to monitor respiratory wellness. Solutions include AudibleHealth AI designed to screen for specific respiratory illnesses including COVID-19, Pneumonia, Tuberculosis and FCV Sentinel which monitors those with chronic respiratory conditions. To learn more about RAIsonance Inc., please visit RAIsonance AI. or FCV Wellness.
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
RAIsonance Announces New Clinical Validation Results for TB, COVID, Pneumonia
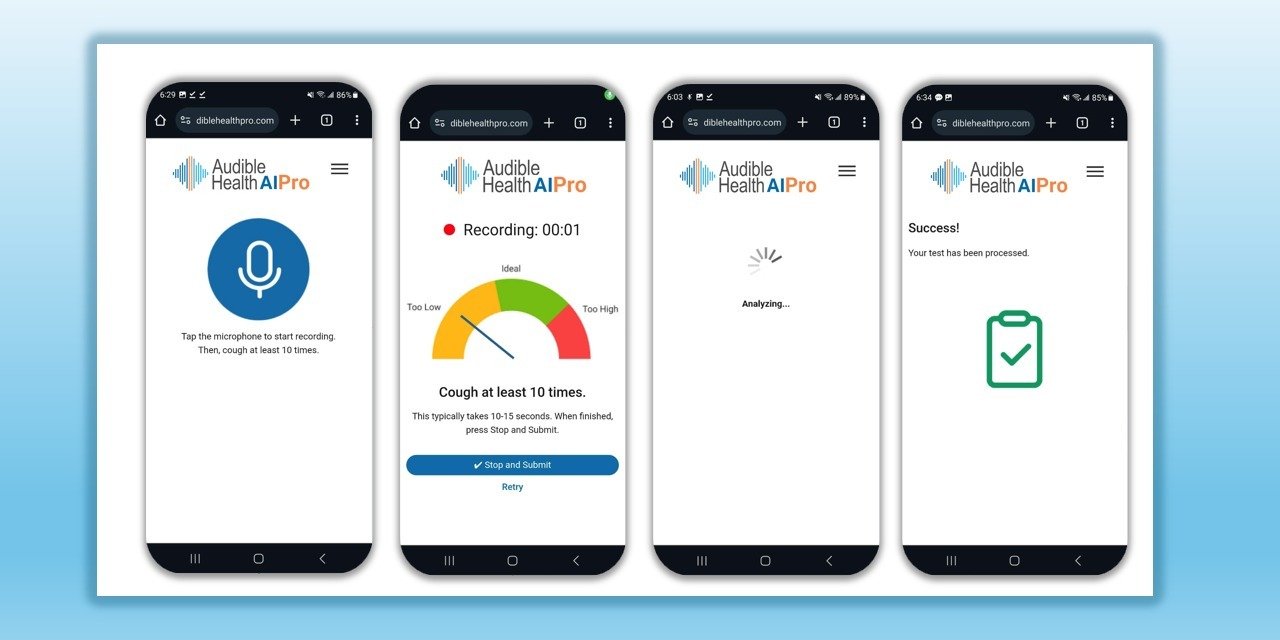
Denver Tech Center, Colorado, May 27, 2025 — RAIsonance, Inc. today announced results from its latest, highly successful field validation of its market-leading respiratory screening solution, used for the detection of Tuberculosis, COVID-19, and Pneumonia in the sound of a person’s cough. The solution, called AudibleHealth AI, uses proprietary artificial intelligence to detect intricate patterns in cough samples that correlate to specific respiratory diseases, and is currently live in 7 countries.
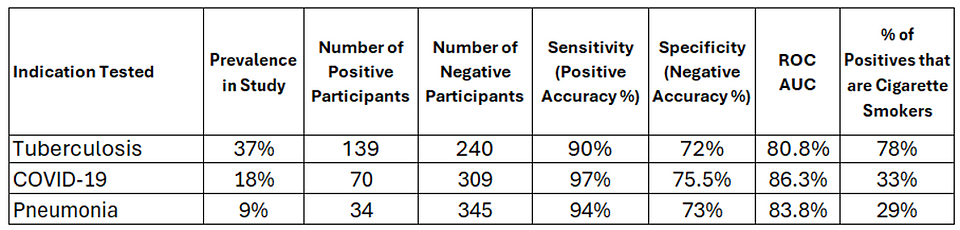
The AudibleHealth AI solution was designed to meet the WHO’s criteria for a screener which calls for 90% sensitivity (positive accuracy) and 70% specificity (negative accuracy). The solution met or exceeded these requirements for Tuberculosis as per the table below. The AudibleHealth AI solutions designed to screen for COVID-19 and Pneumonia were also evaluated on the participant population, and achieved very strong results:
The study wrapped up in May 2025, having taken place in Ukraine. Completed enrollment was 379 participants and was done in collaboration with the Department of Infectious Diseases and Physiatrics at Kharkiv National Medical University, led by Professor Dmytro Butov MD, PhD, ScD. Study enrollment included 100 healthy participants, 139 Tuberculosis positives, and 140 participants with respiratory symptoms that were ultimately found to be positives for COVID-19, Influenza, Pneumonia, Acute Respiratory Infections, Bronchitis, Emphysema, and Sinusitis. The study was a prospective, multi-site field validation that compared the AudibleHealth AI Clinical Decision Support Software results to Xpert MTB/RIF Ultra PCR testing for the diagnosis of Tuberculosis.
Other respiratory illness positives in the study were confirmed using various diagnostic modalities including PCR, Chest X-Ray, antigen, and/or blood tests. “Our technology has been in development and rigorous testing for over five years and has performed beautifully in all five of our clinical studies. This particular study was our most ambitious yet, and was designed to be an especially challenging test of the overall accuracy and disease distinguishability of our solution – that’s another reason why we’re so thrilled with these results,” said Kitty Kolding, Co-Founder and CEO at RAIsonance.
Key validation study challenges included:
- The study took place in a challenging environment in Ukraine, in the midst of the war that started in 2022 when Russia invaded the sovereign nation.
- The study took place amongst a population with many other acute respiratory illnesses
- The population exhibited an unusually high incidence of cigarette smoking across categories, but was most pronounced in the TB positives; over 78% of the TB positive participants were self-reported, active cigarette smokers.
- 140 of the enrolled patients were diagnosed with acute respiratory illnesses other than Tuberculosis
- Of the 140 participants who were diagnosed with non-TB respiratory infections, 77 (or 55%) were diagnosed with co-infections, meaning that these participants tested positive for two or more respiratory illnesses
- Even with these hurdles, the AudibleHealth AI solutions met or exceeded the identified performance targets.
“To achieve the performance targets we sought in this study, our solution needed to correctly detect TB positives with at least 90% accuracy, but it also had to correctly detect that these many other positives and healthy patients were TB negatives. Similarly, our COVID-19 and Pneumonia solutions had to correctly detect the positives they encountered with at least 90% accuracy, while correctly assessing the other positives as COVID-19 and Pneumonia negatives respectively. Additionally, more than 20% of the population had at least 2 co-infections. This represented a complex and difficult combination of conditions, but our solution performed beautifully,” said Karl Kelley, Chief Medical Officer at RAIsonance.
“Our solution is designed to rapidly, non-invasively, and inexpensively identify likely positive cases - at scale. Once identified by our solution, these individuals should undergo the standard confirmatory testing and physician evaluation needed to accurately identify an active TB case, including PCR, imaging, microscopy and sputum culture diagnostic modalities. Our role is to filter large numbers of possible TB sufferers to ensure that these expensive confirmatory tests are conducted only on those most likely to have TB. In this way, we can help high burden TB countries, global health organizations, NGOs and charitable organizations focused on TB to use their limited TB testing resources and personnel much more efficiently and cost-effectively. This way, we can help them to avoid spending heavily on the vast majority of cases that are actually negative,” said Kolding.
“Our unique area of expertise is using proprietary, highly advanced, artificial intelligence and machine learning to analyze and interpret the incredibly rich set of data available from cough sounds. To use the solution, an individual coughs into a customized web app on virtually any Android or Apple smart phone for 10 seconds. That recording is then pushed to the company’s cloud-based production environment in Microsoft Azure, with results returned to the clinician or community health worker in approximately 30 seconds, delivering a fast, simple, effective respiratory screening. We’ve also created a very scalable infrastructure enabling us to process over 20,000 screenings per second, so that we can accommodate virtually any volume spikes we encounter anywhere in the world,” explained Mark Fogarty, Chief Technology Officer at RAIsonance.
“Cough sounds – not to be confused with vocal biomarkers – carry enormous amounts of data that RAIsonance has been studying for years. For each cough collected, we analyze more than 5000 distinct data points. And because every respiratory illness or condition creates different impacts in the respiratory system such as inflammation, airway constriction, changes in mucus quantity and characteristics, etc., our data scientists have discovered an efficient, reliable way to train our models to detect these harmonic patterns in the data that correlate to specific respiratory diseases. That’s how we’re able to not just detect a specific disease but also differentiate between conditions like TB and COVID and Pneumonia,” said Kris Hopkins, Chief Product Officer at RAIsonance.
The AudibleHealth AI solution is designed to give providers and practitioners a fast, portable, inexpensive, non-invasive way to assess their patients during one-to-one interactions as well as to identify likely positive cases at scale. It requires no equipment other than a smartphone, no supplies, generates no waste, requires no shipping or warehousing, and works via SMS text message or WhatsApp all over the world. Because the solution works within a mobile browser, no app downloads or software maintenance is required. As a screener, AudibleHealth AI gives providers fast, critical insights to inform care decisions, so they can proceed with appropriate confirmatory testing as needed.
The company’s AudibleHealth AI platform is designed to house more than 20 different screening solutions. At present the company offers solutions for identifying Tuberculosis, COVID-19, Pneumonia and, for the insurance industry, whether or not a person smokes cigarettes. Solutions for Measles, Influenza, and RSV are in development, and there are another 15 additional solutions on the product roadmap including Pertussis, Emphysema, Asthma, Lung Cancer, Congestive Heart Failure, and many more. Practitioners can choose to have their patient screened for one or many different conditions to provide them with as much data as possible as they assess their patients. The AudibleHealth AI screening solutions for TB, COVID-19 and Pneumonia are now available in seven countries with another 10 markets on the roadmap for 2025.
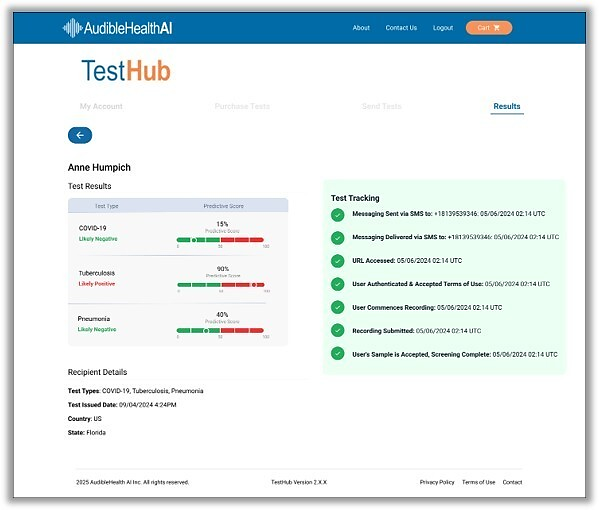
"To bring our solution to market, we’ve activated distributor partnerships with companies all over the world in telehealth, pharmacies, colleges, digital health platforms, and of course clinics, physician practices, and hospitals. We provide all our customers with our web-based solution management system called TestHub – free of charge – to ensure that they can easily send out screening requests and monitor results from anywhere. It’s exciting to work with so many talented, knowledgeable partners in so many markets to introduce this solution to patients they serve," said Kraig Monteferrante, SVP of Global Partnerships for RAIsonance.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based healthcare solutions. RAIsonance companies operate wellness products MyAdvocate and Biometric SoundPass for consumers, the workforce wellness solution FCV Sentinel for the enterprise, and is developing diagnostic solutions for COVID-19, Influenza, Tuberculosis, and RSV. To learn more about RAIsonance, please visit RAIsonance AI. or FCVWellness.com.
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
RAIsonance Launches Inexpensive, Cough-Based TB Solution in 7 Countries
Denver Tech Center, Colorado. Feb 07, 2025 — RAIsonance, Inc. today announced the launch of its AI-powered Tuberculosis clinical decision support software, called AudibleHealth DxPro, in seven countries. This innovative, fully integrated solution uses cough sounds to detect the unique signature of a TB infection in the sound of a person’s cough. The seven launch markets include Guinea, Indonesia, Kenya, South Africa, Rwanda, the United Kingdom, and Zimbabwe.
“Our proprietary technology has been in development and rigorous testing for nearly five years and has performed beautifully in all five of our clinical studies. We are ecstatic to have reached this latest milestone toward our goal of bringing this novel, high-impact technology to high burden TB countries all over the world. We are eager to contribute to the global TB community’s mission of true TB elimination,” said Kitty Kolding, Founder and CEO at RAIsonance.
“Our unique area of expertise is using proprietary, advanced, artificial intelligence and machine learning to analyze and interpret the incredibly rich set of data available from cough sounds. To use the solution, an individual coughs into a customized web app for 10 seconds. That recording is then pushed to the company’s cloud-based production environment in Microsoft Azure, with results returned to the clinician or community health worker in approximately 30 seconds, delivering a fast, simple, effective assessment. We’ve also created a very scalable infrastructure enabling us to process over 20,000 screenings per second, so that we can accommodate virtually any volume spikes we encounter,” explained Mark Fogarty, Chief Technology Officer at RAIsonance.
“Cough sounds – not to be confused with vocal biomarkers – carry enormous amounts of data that RAIsonance has been studying for years. For each cough collected, we analyze more than 5000 distinct data points. And because every respiratory illness or condition creates different impacts in the respiratory system such as inflammation, airway constriction, changes in mucus quantity and characteristics, etc., our data scientists have discovered an efficient, reliable way to train our models to detect these harmonic patterns in the data that correlate to specific respiratory diseases. That’s how we’re able to not just detect a specific disease but also differentiate between conditions like TB and COVID and Flu,” said Karl Kelley, Chief Medical Officer at RAIsonance.
The company’s AudibleHealth Dx platform is designed to house more than 20 different screening solutions. At present the company offers solutions for identifying Tuberculosis, COVID-19, and, for the insurance industry, whether or not a person smokes cigarettes. Solutions for Influenza and RSV are in clinical trials, and there are another 15 additional solutions on the product roadmap including Pertussis, Pneumonia, Emphysema, Asthma, Lung Cancer, Congestive Heart Failure, and many more. Practitioners can choose to have their patient screened for one or many different conditions to provide them with as much data as possible as they assess their patients.
The AudibleHealth DxPro solution for Tuberculosis is designed to give providers and practitioners an entirely new way to assess their patients suspected of a TB infection. The goal of the screening solution is to support active case finding and create powerful new efficiencies in the TB care cascade. The solution was designed to meet or exceed all the latest TPP (Target Product Profile) criteria from the World Health Organization for utility and performance.
“Our solution is designed to rapidly, non-invasively, and inexpensively identify likely positive cases - at scale. Once identified by our solution, these individuals should undergo the standard confirmatory testing and physician evaluation needed to accurately identify an active TB case, including PCR, imaging, microscopy and sputum culture diagnostic modalities. Our role is to filter large numbers of possible TB sufferers to ensure that these expensive confirmatory tests are conducted only on those most likely to have TB. In this way, we can help high burden TB countries, global health organizations, NGOs and charitable organizations focused on TB to use their limited TB testing resources and personnel much more efficiently and cost-effectively. This way, we can help them to avoid spending heavily on the vast majority of cases that are actually negative,” said Kolding.
“Our solution is ideal for low and middle-income countries where TB is the most prevalent. Individuals only need a smart phone and a very basic internet connection to use our technology. And because we designed this as a web app, users don’t need to interact with an app store or download and maintain a mobile app to use the solution. We simply send them a link via SMS or WhatsApp, which launches a mobile browser to allow them to quickly record their cough sounds using our specialized technology,” said Kris Hopkins, Chief Product Officer at RAIsonance.
“To bring our solution to market, we’re activating partnerships with local companies in telehealth, pharmacies, digital health, and of course clinics and hospitals. It’s exciting to work with these talented, knowledgeable partners to introduce this solution to markets they understand,” said Kraig Monteferrante, SVP of Global Partnerships for RAIsonance.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based healthcare solutions. RAIsonance companies operate wellness products MyAdvocate and Biometric SoundPass for consumers, the workforce wellness solution FCV Sentinel for the enterprise, and is developing diagnostic solutions for COVID-19, Influenza, Tuberculosis, and RSV. To learn more about RAIsonance, please visit RAIsonance AI.
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
RAIsonance Releases Results from AudibleHealth Dx Validation Study for Tuberculosis
Greenwood Village, CO, November 30, 2023 – RAIsonance, Inc., a group of AI-powered technology solutions companies, today reported the results of its AudibleHealth Dx clinical validation study. The purpose of the trial was to evaluate the AudibleHealth Dx Software as a Medical Device (SaMD) using Forced Cough Vocalization (FCV) in the diagnosis of active Tuberculosis.
Clinical Study Results
The submission comes after completion of the Clinical Validation study which included 214 participants, consisting of 75 Tuberculosis positive cases and 139 TB negative cases. The study was a retrospective, multi-site, trial that compared the AudibleHealth Dx COVID-19 test to sputum cultures and the GeneXpert RT-PCR.
For the clinical validation study, the AudibleHealth Dx SaMD's ability to correctly detect active Tuberculosis when compared to the BioFire RP2.1 Panel (the first FDA de novo-authorized test for COVID-19). The BioFire RP2.1 Panel runs on the BioFire® FilmArray® Torch and the BioFire® FilmArray® 2.0 Systems in laboratories certified to perform CLIA high-complexity or moderate complexity tests.
When compared to this RT-PCR test and subsequent sputum culture testing, the AudibleHealth Dx has results demonstrating a Positive Percent Agreement (PPA) of 91.2% and a Negative Percent Agreement (NPA) of 75.22%.
Study participants included males and females aged 18 and older who presented for elective, outpatient COVID-19 RT-PCR testing and met the indications for use for the RT-PCR nasal swab test for COVID-19 using the comparator test and AudibleHealth Dx. All participants stated their willingness to comply with all trial procedures, and informed consent was obtained prior to testing.
The validation study included symptomatic and asymptomatic COVID-19 patients as well as healthy subjects who each utilized the AudibleHealth Dx SaMD device on a mobile phone and then immediately were swabbed with an RT-PCR test.
Usability Analysis Results
In addition to evaluating the NPA and PPA of the device, the company also conducted a separate, comprehensive Usability Analysis. Total usability enrollment was 443 participants. Participants included those between the ages of 18 and 88 as well as notable diversity in both ethnicity/race and educational level. Key Usability Analysis outcomes:
- 97% of participants completed the screens of the application completely independently.
- Over 90% felt confident they would know how to receive their results at home.
- 91% felt they would know how to handle a positive or a negative result correctly.
- 97.7% responded that they had a very easy or easy overall experience using the app.
- 98.4% responded that the app screens were very easy or easy to understand.
- 97.5% stated they would be very likely or likely to choose to take an AudibleHealth Dx test at home over other testing options.
- Average time to complete the test, from beginning the registration steps to the delivery of results, was 5.39 minutes.
- Overall successful test completion rate was 99.39%
Background
The use of signal data signatures is common practice for biometric authentication and other means but has not yet been used within the medical community. A forced cough vocalization (FCV), the sound produced when a person intentionally coughs, is unique to each individual and also takes on unique qualities if the individual has certain illnesses, such as COVID-19. RAIsonance has taken this knowledge and the field of artificial intelligence and machine learning (AI/ML) to design and develop a SaMD that uses these unique signatures to diagnose COVID-19 illness.
A Complete Solution
The device, called the AudibleHealth Dx, is designed to diagnose COVID-19 by analyzing the sound of a person’s forced cough vocalization using artificial intelligence. The user interface takes the form of a mobile app. Once the user downloads and registers on the app, they cough 4 to 6 times into their mobile phone, and in about 2 minutes after submitting a cough, they receive their test result in the app. The SaMD approach allows for fast and easy COVID-19 testing with no swabs, no lines, no waste, no expiration dates, and no waiting.
The device is designed so that results are also pushed to a comprehensive Patient Portal where users can learn more about the test, the science, and current public health recommendations regarding COVID-19. Thanks to a technical integration with the Association of Public Health Laboratories (www.APHL.org), the platform is also designed to automatically send any positive results to local public health authorities in all 50 states and 4 U.S. territories.
In addition to the mobile application, the company has also designed a web-based, enterprise class, fully integrated testing solution platform called TestHub, designed for high-volume purchasers. Businesses, employers, universities, sports teams, organizations, and government agencies will be able to use TestHub to acquire any quantity of tests, if authorized by the Food and Drug Administration (FDA), and distribute those tests in any quantities to their users, either once or on a recurring basis, with just a few clicks.
The TestHub dashboard is also designed to enable these high-volume customers to monitor test usage and results to manage risks and support whatever testing protocols they may have in place. In response to the evolving nature of the COVID-19 pandemic, this seamless platform is nimble and versatile enough to flex with policy changes and infection rates.
Of the results, RAIsonance Founder and CEO, Kitty Kolding, said, “We are incredibly proud of the results of these studies. We spent two years refining this technology and building the device itself and were met with dozens of significant challenges along the way. And to ensure that this test can meet the complex testing needs of organizations, we also built a fully integrated, easy to use ecosystem to ensure that users– individuals, businesses, medical professionals, universities, and government agencies – can experience the full benefits of a totally digital COVID-19 testing solution. The combination of our convenient, mobile-app testing interface and our TestHub portal for high-volume purchasers, we believe, is nothing short of revolutionary.”
“We are delighted at the results of this study, which was the culmination of two years of intense work and intentional design. As a physician, the utility of a testing solution like this is a dramatic step forward in terms of scale, innovation, ease of use, convenience, and efficiency. We believe it will be an impressive and impactful tool for mitigating the spread of COVID-19, if authorized by FDA,” added Karl Kelley, MD, FAADM, RAIsonance’s Chief Medical Officer.
Mona Kelley, Chief of Clinical and Regulatory Affairs for RAIsonance is also pleased with the results. “Given the novelty of the test, understanding the usability of the test was essential. We are very excited about our Usability Analysis results and how well the device performed in that regard.”
“I am so proud of the SaMD that we’ve built. Not only is it a complete solution, but it’s also almost infinitely scalable, cost-effective, and user-friendly. Seeing these study results is incredibly gratifying as the validation for all of our work over the past two years,” said Mark Fogarty, Chief Technical Officer (CTO) and Chief Biotechnical Engineer (CBTE).
Lead Series A investor and Chairman of the Board, Rudy Karsan, details AudibleHealth Dx’s next steps. “Our SaMD has the potential to add a powerful new tool to conduct COVID-19 testing. With this novel technology, we can facilitate mass testing without supply-chain headaches, inventory issues and excessive costs. We expect this integrated platform, if authorized by FDA, to become indispensable for businesses, healthcare providers, universities, government bodies and any other type of organization that would benefit from routine COVID-19 testing. The scalability is virtually limitless.”
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based healthcare solutions. RAIsonance companies operate wellness products MyAdvocate and Biometric SoundPass for consumers, the workforce wellness solution FCV Sentinel for the enterprise, and is developing diagnostic solutions for COVID-19, Influenza, Tuberculosis, and RSV. To learn more about RAIsonance Inc., please visit RAIsonance.ai
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
RAIsonance Launches the Air Quality Human Impact Program
Greenwood Village, Colorado, August 29, 2023. RAIsonance today announced the release of its Air Quality Human Impact Program (IQHIP) for the US market. The specialized biometric monitoring system tracks and reports on the impact of poor air quality on the human respiratory system. Using proprietary AI technology and a mobile app interface, the platform analyzes intentional coughs from users all over the United States to detect negative impacts to the human respiratory system.
The science of air quality measurement is mature, sophisticated, and accurate. Air quality sensors can detect pollutants and toxins with very good precision. Access to the air quality data for those sensors is readily available from reputable organizations such as BreezeoMeter, IQAir, PurpleAir, and the EPA’s AirNow.gov.
"Until now there has been no comprehensive, science-based way to understand the impact of poor air quality on our bodies, or for how long those impacts last”, said Kitty Kolding, CEO of RAIsonance. “Our biometric FCV Sentinel technology is designed to measure these impacts with precision. Our 24/7 reporting platform will make these critical impact reports as ubiquitous as air quality alerts, giving all of us accessible insight into potential health risks affecting ourselves and our loved ones."
According to the EPA, the most common pollutants are found all over the United States and include particulate matter (often referred to as particle pollution), ground-level ozone, carbon monoxide, sulfur dioxide, nitrogen dioxide, and lead. These pollutants can harm human health, harm the environment, and cause property damage.
According to the Canadian Wildfire Information System, 33.8 million acres have burned in Canada so far this year, which is almost twice Canada’s previous annual record set in 1989. These fires have created exceptionally bad air quality conditions at various times across the US. Poor air quality leaves those with existing respiratory problems open to real harm, and can create new health problems for otherwise healthy individuals of all ages.
"We want to give people easy-to-use, valuable tools to help understand health risks specific to them. When people understand how poor air quality is affecting them personally, they are far better prepared to take action that protects themselves, their families, and their communities," said Kris Hopkins, Chief Product Officer at RAIsonance.
To measure these impacts, RAIsonance is recruiting users to join the AQHIP. Users will be given free, unlimited access to RAIsonance’s FCV Sentinel mobile app and cloud based analysis platform to track their personal health impacts. Participants are asked to cough into the FCV Sentinel mobile app at least once a day. Their data will be analyzed and correlated with air quality measurements, feeding live dashboards and the AQHIP alert system.
"Our AQHIP empowers people everywhere to better understand threats to their health, so they can proactively take steps to protect themselves and their loved ones. While our first launch is in the US, we’re expanding this program globally over the next 6 months”, said Kolding. “We hope people everywhere world will join this movement to monitor and protect human respiratory health."
To join the program, sign up at https://AQHIP.com/JOIN
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based healthcare solutions. RAIsonance companies operate wellness products MyAdvocate and Biometric SoundPass for consumers, the workforce wellness solution FCV Sentinel for the enterprise, and is developing diagnostic solutions for COVID-19, Influenza, Tuberculosis, and RSV. To learn more about RAIsonance, please visit RAIsonance AI.
About FCV Sentinel
FCV Sentinel tracks FCV scores to provide an early warning if problematic changes are identified. FCV Sentinel is a threat-agnostic early warning system, based on RAIsonance’s proprietary technology: Forced Cough Vocalization (FCV) analysis. This tool is designed to detect subtle changes that may be the result of exposure to a pathogen, chemical agent, or environmental toxin experienced by the user. These changes are reported as an FCV Impact Score, with higher numbers indicating little or no change. Lower numbers indicate notable differences that may warrant further attention. For businesses, groups and organizations, aggregate data is presented on interactive dashboards in near real time, and can be customized to track readiness by group, location, region, etc. To learn more about FCV Sentinel, please visit FCV Wellness
RAIsonance Investor Contact:
Jamie Mariani, CFO
Email: InvestorRelations@raisonance.ai
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
Raisonance Releases New Fall 2023 Workforce Risk Forecast
Greenwood Village, Colorado. August 21, 2023 — RAIsonance today announced the release of its Fall 2023 Workforce Wellness Risk Forecast Report. This specialized forecast delivers comprehensive insights into rising health risks likely to affect workforce health and productivity this fall, across industries and sectors. The first in a series, these reports provide employers with a science-based risk forecast to help them better prepare for and mitigate potential productivity disruptions resulting from the forecasted incidence of contagious illnesses in the US, Canada, and UK.
"Working in conjunction with one of the most talented biorisk experts in the world, we've devised a critical tool to help employers get a glimpse into the multi-faceted illness environment we may be facing this fall and winter. We believe employers of all sizes need an evidence-based way to anticipate biorisks that can threaten their employees’ health, and their companies' productivity and profitability" said Kitty Kolding, CEO and Founder of RAIsonance, Inc.
The Fall 2023 report cites several specific trends that could create a very challenging environment for employers through the remainder of 2023. These insights are especially critical to employers in categories whose workforce must be on-site, such as construction, healthcare, first responders, manufacturing, mining, warehousing, and the like.
“To support our enterprise clients, we watch these increasing workforce wellness threat indicators very closely,” said Kris Hopkins, Chief Product Officer at RAIsonance. “Our enterprise wellness tracking solution, FCV Sentinel, is expressly designed to mitigate these kinds of threats to productivity, to help employers work through these fluid and challenging conditions.”
According to the US Bureau of Labor Statistics, about “7.8 million workers missed work in January 2022 because they had an illness, injury, or medical problem or appointment, up from 3.7 million in January 2021. About 4.2 million who usually work full-time worked part-time because of an illness, injury, or medical problem or appointment, up from 1.8 million in January 2021.”
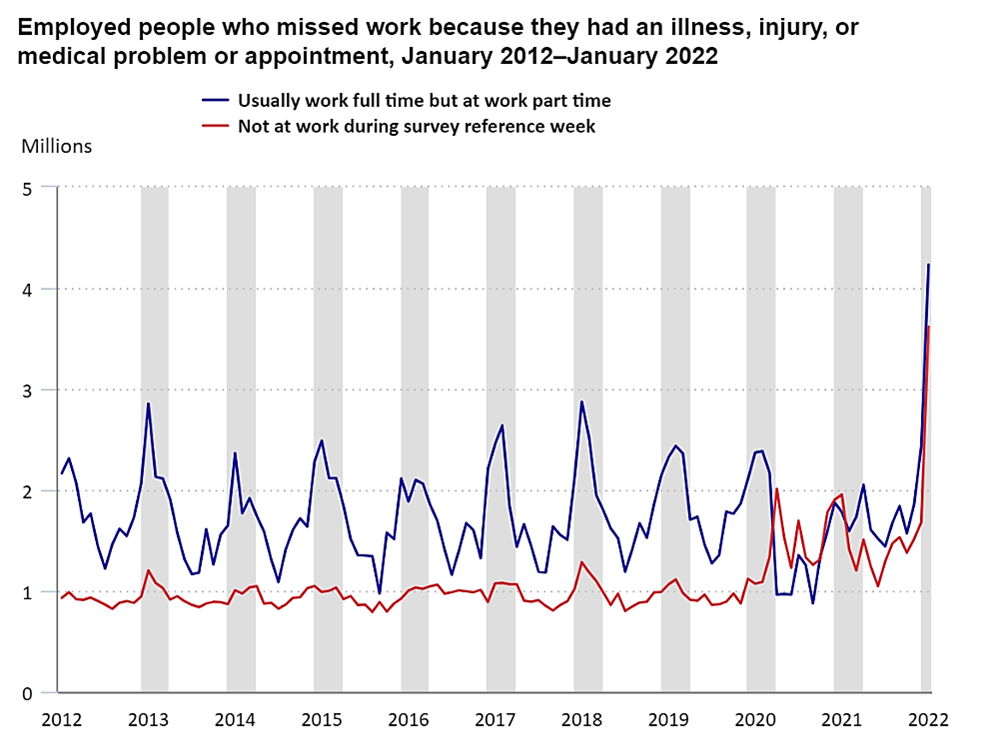
Absenteeism alone costs U.S. companies an estimated $225.8 billion annually, according to the Centers for Disease Control and Prevention (CDC). A Harvard Business Review study suggests that presenteeism can cost U.S. companies up to $150 billion per year. Presenteeism costs include decreased productivity, low morale among other employees, and the potential health risk of communicable disease spread. The relentless toll of these costs can substantially erode a company's profit margin, emphasizing the necessity for effective health and risk management strategies in the workforce.
Key Insights from the Fall 2023 Workforce Wellness Risk Forecast Report
- The report notes the emergence of a new sub-lineage of Omicron, EG.5.1, which will drive a new wave of Covid-19 infections. This wave began at the start of August and will likely peak in early to mid-September in both the US, Canada, and the UK, with different spikes in different markets. The wave will be similar in magnitude to the 2022 – 2023 winter waves experienced in North America and the UK.
- In addition, a 2nd generation sub-lineage of BA.2 known as BA.2.86 has been detected in Denmark, London, USA, and Israel. While case numbers are currently very small, the large number of mutations present in this sub-lineage and the fact it has been detected in four distinct geographic locations raise the possibility that this sub-lineage may cause a new global wave of infections.
- Increased levels of ill-health from a combination of Influenza, RSV, COVID-19, Group A Strep, Adenovirus and a handful of other infectious diseases will increase levels of staff absences.
- Severe cases of Long Covid (LC) will continue to accumulate, creating a new group of individuals who are physically unable to work.
- Neurocognitive dysfunction (“Brain Fog”) will adversely affect the job performance of approximately 25% of those individuals who are infected with SARS-CoV-2. Brain fog can last for several weeks after all other symptoms of acute COVID -19 illness resolve, and thus will negatively impact individual productivity long after an individual returns to work after a COVID -19 absence.
- These workforce shortages will also cause some supply chain problems, including for some medications, specifically oral formulations of common broad-spectrum antibiotics. Other medications, such as short-acting bronchodilators for asthma and COPD, are also likely to have supply chain interruptions.
- The immune dysregulation effect of COVID -19 will lead to significant winter waves of RSV and Strep A in children. This may lead to a small but significant number of temporary school closures, and parents requiring time off work to care for sick children. These diseases are likely to occur at similar rates as 2022.
The full report also details related Societal Risks and Available Mitigations. To obtain the full report without charge, please
- Go to: Risk Forecast Download
- Or Email: RAIsonance Sales
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based healthcare solutions. RAIsonance companies operate wellness products MyAdvocate and Biometric SoundPass for consumers, the workforce wellness solution FCV Sentinel for the enterprise, and is developing diagnostic solutions for COVID-19, Influenza, Tuberculosis, and RSV. To learn more about RAIsonance Inc., please visit RAIsonance.ai
RAIsonance Media Contact:
Public Relations Manager
Email: Press@raisonance.ai
RAIsonance, Inc. Announces BARDA Contract to Continue Development of the AudibleHealth Dx Diagnostic SaMD for COVID-19 and Influenza Through AI-Powered Cough Analysis
RAIsonance’s Forced Cough Vocalization (FCV) Signature Analysis Technology is Designed to Diagnose Respiratory Illnesses Via a Cloud-Based Diagnostic Platform and a Mobile App User Interface
DENVER, April 12, 2023 – RAIsonance, Inc., the leader in artificial intelligence-powered analysis of FCV, announced today it has been awarded a contract from the Biomedical Advanced Research and Development Authority (BARDA), a component of the Administration for Strategic Preparedness and Response (ASPR), within the U.S. Department of Health and Human Services (HHS), a U.S. government agency.
The contract consists of just over $749,000 allocated to the continued development of RAIsonance’s AudibleHealth Dx Software as a Medical Device (SaMD). The device is designed to analyze a user’s Forced Cough Vocalization (FCV) in the diagnosis of illness, with the current focus on both COVID-19 and Influenza. The cloud-based artificial intelligence processing platform uses a mobile app interface for cough collection and is designed to deliver test results in about 2 minutes within the app. The primary objectives of the contract are to complete the execution of the clinical validation studies for COVID-19 and Influenza and to complete a de novo submission to the U.S. Food and Drug Administration before the end of 2023.
“We are elated to have BARDA’s support. Their strategic guidance and extensive expertise will speed our efforts to deliver the best possible diagnostic SaMD to market and help ensure that we fulfill our mission to meaningfully impact global health with such an innovative diagnostic solution,” said Kitty Kolding, Co-Founder and CEO at RAIsonance.
RAIsonance’s SaMD is designed to use only software to diagnose disease, without any wet or biological samples of any kind. The non-invasive test requires just 10 intentional coughs, submitted via a uniquely devised mobile application. Tests can be delivered on-demand electronically, are independent of supply chains, and are not limited by shelf-life expiration. The SaMD is scalable – able to process 20,000 tests per second – an ideal solution for large organizations, colleges and universities, and government agencies.
This award is one component of BARDA's rapidly expanding DRIVe Medical countermeasures portfolio; visit https://drive.hhs.gov to learn more.
“This project has been supported in whole or in part with federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; Biomedical Advanced Research and Development Authority (BARDA), under contract number 75A50123C00015.”
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets. Originally funded with a grant from the National Science Foundation, RAIsonance has developed breakthrough technology innovations that use signal data signature recognition as a biomarker for human medical diagnostics and to support ground-breaking safety applications. To learn more about RAIsonance, please visit RAIsonance AI.
RAIsonance Investor Contact:
Jamie Mariani, CFO
Email: InvestorRelations@raisonance.ai
RAIsonance Media Contact:
Denise Covington
Email: Press@raisonance.ai
RAIsonance, Inc. Launches AI-Powered Smoker Recovery App
MyAdvocate Product Tracks Progress Upon Quitting and Delivers a Personalized Biometric Score for Each User’s Respiratory Wellness Journey
Denver, CO, April 11, 2023 – RAIsonance, Inc., the leader in artificial intelligence-powered analysis of Forced Cough Vocalization (FCV), today announced the launch of two new apps for iOS and Android mobile devices. The apps, MyAdvocate Smoker Recovery and MyAdvocate Vape Recovery, use AI to support cessation by tracking each user’s personal progress, and providing them with personal biometric cough score analyses that measure their journey back to respiratory health. The apps are free to download on the App Store and Google Play, and specially priced 6-month and 12-month packages can be purchased from the MyAdvocate storefront.
“Kicking a nicotine habit is extremely difficult, no matter who you are” said Kitty Kolding, CEO and Co-Founder of RAIsonance, Inc., said, “On average, people make 5 to 7 attempts to quit before they actually succeed. We created this product because we know that being able to see your personal progress and improvement makes a huge difference in a person’s motivation to keep going, and quit for good. Using artificial intelligence, we can provide users with an incredibly detailed, completely customized understanding of their progress back to health.”
In the US, more than 30 million adults smoke cigarettes and more than 2.5 million high school and middle school kids use e-cigarettes. And, at any given moment, approximately 65% of smokers say they want to quit. Recent studies by NIH show that “mobile phone-based interventions, interactive and tailored internet-based interventions, … had modest but significantly increased benefits to smoking cessation at 6 or more months”.
To set up MyAdvocate, the mobile app guides a user through recording several sets of intentional coughs called FCVs. From those recordings, MyAdvocate uses AI to create a baseline - a composite of the user’s FCV signature as unique as their fingerprint. This baseline is a multi-dimensional representation of the imprint representing a user’s respiratory function. With this baseline in place, a user can check their progress any time by coughing again into the app. The MyAdvocate AI compares the current cough to the baseline, and shows an Improvement Score in about 2 minutes, right in the app.
Both apps are designed to work alongside any other smoke or vape cessation products or programs. Similar to dieting, no matter what diet program a person might use, they always use a scale to measure their progress. MyAdvocate is exactly that: the ultimate biometric measure of progress back to health. In testing for more than 2 years, the MyAdvocate FCV tracking technology is the only system that uses AI-powered deep science to track the changes to a user’s unique FCV.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets. Originally funded with a grant from the National Science Foundation, RAIsonance has developed breakthrough technology innovations that use signal data signature recognition as a biomarker for human medical diagnostics and to support ground-breaking safety applications. To learn more about RAIsonance, please visit RAIsonance AI.
RAIsonance Investor Contact:
Jamie Mariani, CFO
Email: InvestorRelations@raisonance.ai
RAIsonance Media Contact:
Denise Covington
Email: Press@raisonance.ai
RAIsonance and Soter Technologies Partner to Bring
FCV Signature Analysis to Enhance Vaping and Smoking Cessation
Ronkonkoma, NY and Denver, CO, March 22, 2023 – RAIsonance Inc., a family of companies dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets has partnered with Soter Technologies, the Long Island-based creators of the world’s first vape detection and alert system for schools. The companies will work together to bring MyAdvocate, a Forced Cough Vocalization (FCV) Signature Analysis technology app, to schools and parents to support the reduction of youth vaping and smoking.
Parents and school administrators across the United States have been looking for tools and approaches to stop the youth vaping epidemic. School administrators in particular want to find ways to help students rather than punishing them when they are addicted to nicotine. MyAdvocate gives parents and school leaders mobile technology that young people can connect with and use to monitor their progress as they work to break their nicotine addiction.
MyAdvocate for Vape Recovery and Smoking Recovery is a completely new, biometric tracking app that detects and measures the respiratory improvements users achieve while quitting smoking or vaping. Using artificial intelligence, MyAdvocate technology compares Forced Cough Vocalization (FCV) signatures, which are intentional coughs, to the user’s previously created baseline. With each new cough, MyAdvocate generates a new score that shows the recovery progress.
To create a baseline, users record several sets of FCVs. From those recordings, MyAdvocate uses AI to create a baseline that is as unique as a fingerprint. This baseline is a multi-dimensional composite consisting of dozens of unique cough features from each cough. Each new cough is then compared to this baseline and scored, to show the user’s progress toward recovery. The MyAdvocate product line works on both Android and Apple smartphones, giving schools, parents, and young people an additional set of smart tools to help them stop vaping and smoking.
"Our proprietary AI analyzes these biometric FCV signals, creating a personalized way to capture, track and monitor progress. Young people are drawn to mobile technology and it is our goal to give them a tool that is easy to use, and helps them see the result of their efforts to quit. Providing frequent, direct, and personal feedback is a proven approach to supporting this very difficult journey. Partnering with Soter Technologies is a big win for us – they are a leader in this space and have done tremendous work in supporting young people, their families, and their schools in this critical initiative. We are so proud to be working with them," said Kitty Kolding, CEO and Founder, RAIsonance Group of Companies.
Soter’s experience in the K-12 market, mobile application and backend monitoring technology have been paired with RAIsonance’s AI cough analyzer technology. By combining their technology, MyAdvocate for Vape Recovery app users have real time tracking of their cessation efforts.
"Youth smoking continues to be a problem and the student vaping epidemic is out of control across the USA. Millions of students from middle schools and high schools are vaping, and school administrators need solutions that will help students who are addicted instead of punishing them. Education and awareness help reduce vaping and smoking, but the process of breaking the addiction cycle is difficult and painful for many, especially young people. MyAdvocate for Smoking Recovery and Vape Recovery is a perfect addition to the products and services we offer. With this technology as a key part of anti-vaping efforts, I am certain we will help more young people break the nicotine addiction cycle and quit vaping and smoking," said Derek Peterson, CEO Soter Technologies.
MyAdvocate will be added to Soter Technologies FlySense vape detection and alert system and "NO VAPE" educational awareness program which has been proven to reduce vaping in schools by focusing on the health hazards.
Post-pandemic vaping has reemerged in schools. More and more students are vaping and each vape liquid pod contains the same amount of nicotine as a pack of cigarettes in addition to other harmful chemicals.
For more information about the Vape Recovery app from RAIsonance, please visit our LinkedIn Showcase page
For more information about Soter Technologies, visit Soter Technologies
- END -
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets. Originally funded with a grant from the National Science Foundation, RAIsonance has developed breakthrough technology innovations that use signal data signature recognition as a biomarker for human medical diagnostics and to support ground-breaking safety applications. To learn more about RAIsonance, please visit RAIsonance AI
About Soter Technologies
Based in Ronkonkoma, New York, Soter Technologies is committed to protecting the health and wellbeing of students and the general public with advanced technology and creative solutions that fits today’s marketplace and needs. Using advanced sensor, IoT, and software technology, Soter Technologies develops and delivers innovative solutions in two distinct and unique product lines FlySense® and SymptomSenseTM. FlySense® offers environmental intelligence, which makes the world a safer and healthier place, from schools to enterprises to public spaces. The FlySense® Vaping and Elevated Sound Detector is the first in the world to introduce a vape and bullying detection and alert system for schools. SymptomSense™ products are designed to detect illness and protect facilities. Soter Technologies’ name is inspired by Greek mythology wherein Soter is the personification of safety, deliverance, and preservation from harm. For more information about the company and its services and products, visit:
Soter Technologies
RAIsonance Investor Contact:
Jamie Mariani, CFO
Email: InvestorRelations@raisonance.ai
RAIsonance Media Contact:
Denise Covington
Email: Press@raisonance.ai
Soter Media Inquiries:
Bill Corbett
Email: wjcorbett@corbettpr.com
Tel: (516)775-0435
Long COVID Tracking App Enrolling for Beta Test
Denver, CO, March 15, 2023 – RAIsonance, Inc., announces the beta testing phase of its innovative, AI-powered tracker for sufferers of Long COVID. Part of the MyAdvocate line of Forced Cough Vocalization (FCV) wellness products, this platform was specially developed to track the unique and complex symptoms experienced by Long COVID sufferers, and to track changes to their FCV score – a key respiratory wellness metric.
“We are thrilled to welcome beta testers to experience our new MyAdvocate for Long COVID application. COVID has been a terrible scourge for so many people around the world – and for some people, COVID never goes away. That’s who we made this product for.” Kitty Kolding, CEO of RAIsonance. “By capturing and analyzing this data-rich FCV signal, our tools can provide Long COVID sufferers with a new, trackable biometric data point as they continue on their journey to recovery.”
The product’s user interface takes the form of a mobile app, which communicates with the cloud-based FCV processing and analytics system. The system enables users to create their unique Baseline FCV profile by coughing into the application multiple times on the first day. Then, users can cough again any time and have the system compare their new cough to their baseline, yielding an FCV change score. This score helps users understand how their FCV score changes over time, and even throughout the day as their symptoms might worsen or subside.
Users can also easily input their symptoms with a few clicks using the app’s symptom diary. The app provides trend analysis and other insights to help users get a clearer picture of their situation and share these details with their healthcare providers if they wish.
The beta testing period opens on March 15, 2023, and is free to Long COVID sufferers. Click HERE for more information about MyAdvocate for Long COVID, and to join the beta testing group, click HERE.
RAIsonance Develops FCV Signature Analysis Technology for Health, Wellness
Denver, CO, March 13, 2023 – RAIsonance, Inc., the leader in artificial intelligence powered analysis of Forced Cough Vocalization (FCV), announces expansion into new wellness product categories. This proprietary technology focuses on FCV signature analysis, which uses these intentional coughs as a rich source of biometric and biomarker data to detect changes to an individual’s cough sound signatures, tracking a key respiratory wellness metric.
RAIsonance has incorporated this FCV Wellness technology into 3 new product lines launching over the next 60 days:
- FCV Sentinel is a threat-agnostic early warning system that detects changes that may be the result of a pathogen, chemical agent, or environmental toxin experienced by the user.
- MyAdvocate is designed specifically to assist individuals for ongoing conditions that users wish to track such as Long COVID, or to track improvement such as once a person has quit smoking or vaping.
- Biometric SoundPass operates like a “check engine” light, alerting the user that an out-of-range FCV change has been detected and provides a “first alert” signal.
Each of the RAIsonance product lines use a mobile app interface to collect a series of intentional coughs which are processed in their cloud-based FCV analysis system. Guided by the app, these initial coughs are collected over a prescribed period of time to build a complex, multi-dimensional FCV profile or baseline for the user. Once established, the user can cough into the mobile app again anytime, and the powerful Artificial Intelligence (AI) rapidly compares that user’s new cough to their previously established baseline. In about 2 minutes, the results are available in the app, designed to track even the smallest changes.
Learn more about RAIsonance’s line of FCV Signature Analysis products HERE.
Investor Contact:
Jamie Mariani, CFO
Email: InvestorRelations@raisonance.ai
Media Contact:
Denise Covington
Email: Press@raisonance.ai
SOURCE RAIsonance, Inc.
RAIsonance Announces Executive Leadership Appointments
Denver, CO, February 14, 2023 – RAIsonance, Inc., a group of Artificial Intelligence – Machine Learning (AI-ML) technology solutions companies, today announced the promotion of Paul McLenaghan to Chief Revenue Officer and the hiring of Kris Hopkins as Chief Product Officer.
Paul McLenaghan, Chief Revenue Officer
Paul McLenaghan joined RAIsonance in July 2021 as SVP of Market Development. He is a market and business development executive with a passion for driving market disruption. Since joining RAIsonance, he has worked with executive leadership to develop strategic relationships, identify new market opportunities, and drive go to market activities across the government, private enterprise, and education sectors.
Prior to RAIsonance, Paul spent close to twenty years in the advertising technology industry. He was most recently with Prohaska Consulting, where he consulted to major ad tech platforms, publishers, and media companies on data strategy and new product strategy. Previously, he spent close to fifteen years at TARGUSinfo and Neustar, where he had a track record of success creating and driving new solutions and lines of business at the intersection of technology, data, and media in executive roles across Product Strategy and Market Development.
Paul completed his MBA at the University of Maryland Robert H. Smith School of Business and has BA degrees in Economics and English from Villanova University. He is a dual citizen of Australia and the United States.
Kris Hopkins, Chief Product Officer
Kris Hopkins is an entrepreneur, inventor, and product leader in software. Previously, Kris has led strategy, product, and engineering as CTO and/or Chief Product Officer at multiple companies including at Perfecta, a cybersecurity, intelligence, and communications technology provider, and led strategic product management for new products at Oracle. As founder and CEO of Newfound Communications, Kris sold the company to Acme Packet (NASDAQ: APKT) and spun out its Software as a Service business into Surgent Networks. A dynamic leader of software product and development teams, Kris brings to RAIsonance 20 years of experience managing mission-critical and high-security software development projects and pipelines. With a focus on the largest enterprise and government customers, his leadership builds robust cloud-based and premise-based software products, SDKs, and APIs for businesses to provide secure and efficient solutions.
Kris is a graduate of Cornell University where he was the recipient of the Dorman Family Award for Entrepreneurship and Personal Enterprise Excellence. He is a dedicated husband, father of two, loves coaching youth sports, and is an active member of his community.
Driving a Revolution in AI-ML Technology for Healthcare
Mr. Hopkins and Mr. McLenaghan join RAIsonance’s executive team at a critical moment in the company’s growth. Founded in March of 2020, the company set out to devise and bring to market the first software only, AI-powered COVID test using only the sound of a person’s cough to render a diagnosis. The device, called AudibleHealth Dx, is designed to diagnose COVID-19 by analyzing the sound of a person’s forced cough vocalization using artificial intelligence.
The user interface takes the form of a mobile app. Once the user downloads and registers on the app, they cough 4 to 6 times into their mobile phone, and in about 2 minutes after submitting a cough, they receive their test result in the app. This all-digital approach allows for fast and easy COVID-19 testing with no swabs, no lines, no waste, no expiration dates, and no waiting. The massively scalable testing platform is capable of processing many thousands of tests per second and is integrated with the company’s TestHub digital test acquisition, distribution, and tracking platform.
The RAIsonance team successfully completed its EUA clinical trials for the COVID test in late 2022 and has filed applications for Emergency Use Authorization in the US and Canada. The company is currently working on its second and third tests on the platform, for Influenza and Tuberculosis.
Of the new hires, RAIsonance Co-Founder and CEO, Kitty Kolding, said, “Kris and Paul are both impressive, accomplished executives. They join our executive team at the perfect moment as we launch several new AI-ML health tech solutions while continuing development on our Software as a Medical Device (SaMD).”
When asked about his role at RAIsonance, Kris Hopkins replied, “Since the onset of the global pandemic, AI and Machine Learning have held great promise for the diagnosis and wellness assessments of respiratory ailments. As Chief Product Officer, I am looking forward to building upon and extending the impressive work by the RAIsonance team. We are in a position to deliver secure, breakthrough products which leverage our experience with AI/ML bio-acoustic markers for the reliable detection of respiratory conditions, leading to improved outcomes and better health equity.”
Paul McLenaghan said about his promotion to Chief Revenue Officer, “I joined RAIsonance in 2021 to contribute to the company’s mission to have a positive impact on global health and wellness. It has been a privilege to work alongside such a talented and passionate team these past 18 months to develop a suite of innovative, 100% digital diagnostic and wellness solutions that are affordable, reliable, and scalable. I look forward to working with Kitty and the rest of the team to deliver our solutions to health providers, public health officials, employers, and most importantly - individuals in the US and globally.”
About RAIsonance, Inc.
RAIsonance, Inc., a family of AI-powered technology solution companies headquartered in the Denver Tech Center, is focused on meeting today’s critical health and safety challenges. Founded in March of 2020, AudibleHealth AI, Inc. is the SaMD division of RAIsonance, specializing in AI/ML diagnostic solutions and platforms. Originally funded by an SBIR grant by the National Science Foundation, the team of medical, artificial intelligence, technology, and medical device specialists focuses on developing leading-edge AI/ML-based, scalable, cost-effective diagnostic products across a range of acute and chronic health conditions.
For more information visit us at RAIsonance AI and AudibleHealth AI
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance Submits EUA Application to FDA for AudibleHealth Dx
Greenwood Village, CO, September 26, 2022 – RAIsonance, Inc., today announced that it has submitted an application to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of its AudibleHealth Dx Software as a Medical Device (SaMD). The device is designed to analyze a user’s Forced Cough Vocalization-Signal Data Signature (FCV-SDS) in the diagnosis of COVID-19 illness. Results in the clinical validation study demonstrated a Positive Percent Agreement (PPA) of 84.39% and a Negative Percent Agreement (NPA) of 85.09% when compared to a reverse transcription-polymerase chain reaction (RT-PCR) test.
“I am beyond thrilled to have submitted our EUA Application for AudibleHealth Dx.” said RAIsonance Founder and CEO, Kitty Kolding. “If authorized, we will immediately bring to market a convenient, accessible, inexpensive, and innovative COVID-19 test that will truly allow everyone to test as often as they wish.”
About the Clinical Trial
The submission comes after completion of the Clinical Trial conducted in Florida, and enrolled 515 participants, which included 65 COVID-19 positive cases and 450 COVID-19 negative cases for a prevalence of 12.6%. The study was a prospective, multi-site, non-inferiority trial that compared the AudibleHealth Dx COVID-19 test to a highly sensitive de novo-authorized RT-PCR test to detect COVID-19 infections.
For the clinical validation study, the AudibleHealth Dx SaMD's ability to correctly diagnose COVID-19 was compared to the BioFire RP2.1 Panel (the first FDA de novo-authorized test for COVID-19). The BioFire RP2.1 Panel runs on the BioFire® FilmArray® Torch and the BioFire® FilmArray® 2.0 Systems in laboratories certified to perform CLIA high-complexity or moderate complexity tests.
When compared to this RT-PCR test, the AudibleHealth Dx has results demonstrating a Positive Percent Agreement (PPA) of 84.39% and a Negative Percent Agreement (NPA) of 85.09%.
Study participants included males and females aged 18 and older who presented for elective, outpatient COVID-19 RT-PCR testing and met the indications for use for the RT-PCR nasal swab test for COVID-19 using the comparator test and AudibleHealth Dx. All participants stated their willingness to comply with all trial procedures, and informed consent was obtained prior to testing.
The validation study included symptomatic and asymptomatic COVID-19 patients as well as healthy subjects who each utilized the AudibleHealth Dx SaMD device on a mobile phone and then immediately were swabbed with an RT-PCR test.
Usability Analysis Results
In addition to evaluating the NPA and PPA of the device, the company also conducted a separate, comprehensive Usability Analysis. Total usability enrollment was 443 participants. Participants included those between the ages of 18 and 88 as well as notable diversity in both ethnicity/race and educational level. Key Usability Analysis outcomes:
- 97% of participants completed the screens of the application completely independently.
- Over 90% felt confident they would know how to receive their results at home.
- 91% felt they would know how to handle a positive or a negative result correctly.
- 97.7% responded that they had a very easy or easy overall experience using the app.
- 98.4% responded that the app screens were very easy or easy to understand.
- 97.5% stated they would be very likely or likely to choose to take an AudibleHealth Dx test at home over other testing options.
- Average time to complete the test, from beginning the registration steps to the delivery of results, was 5.39 minutes.
- Overall successful test completion rate was 99.39%
Mona Kelley, Chief of Clinical and Regulatory Affairs for RAIsonance is also pleased with the results. "Given the novelty of the test, understanding the usability of the test was essential. We are very excited about our Usability Analysis results and how well the device performed in that regard."
Background
The use of signal data signatures is common practice for biometric authentication and other means. A forced cough vocalization (FCV), the sound produced when a person intentionally coughs, is unique to each individual and also takes on unique qualities if the individual has certain illnesses, such as COVID-19. RAIsonance has taken this knowledge and the field of artificial intelligence and machine learning (AI/ML) to design and develop a SaMD that uses these unique signatures to diagnose COVID-19 illness.
A Complete Solution
The device, called the AudibleHealth Dx, is designed to diagnose COVID-19 by analyzing the sound of a person’s forced cough vocalization using artificial intelligence. The user interface takes the form of a mobile app. Once the user downloads and registers the app, they cough 6 to 10 times into their mobile phone, and in about 2 minutes, after submitting a cough, they receive their test result in the app. The (SaMD) approach allows for fast and easy COVID-19 testing with no swabs, no lines, no waste, no expiration dates, and no waiting.
The device is designed so that results are also pushed to a comprehensive Patient Portal where users can learn more about the test, the science, and current public health recommendations regarding COVID-19. Thanks to a technical integration with the Association of Public Health Laboratories (www.APHL.org), the platform is also designed to automatically send any positive results to local public health authorities in all 50 states and 4 U.S. territories.
In addition to the mobile application, the company has also designed a web-based, enterprise class, fully integrated testing solution platform called TestHub, designed for high-volume purchasers. Businesses, employers, universities, sports teams, organizations, and government agencies will be able to use TestHub to acquire any quantity of tests, if authorized by the FDA, and distribute those tests in any quantities to their users, either once or on a recurring basis, with just a few clicks.
The TestHub dashboard is also designed to enable these high-volume customers to monitor test usage and results to manage risks and support whatever testing protocols they may have in place. In response to the evolving nature of the COVID-19 pandemic, this seamless platform is nimble and versatile enough to flex with policy changes and infection rates.
About the AudibleHealth Dx submission and the TestHub platform, Mark Fogarty, Chief Technical Officer (CTO) and Chief Biotechnical Engineer (CBTE) said, “This submission is the next step towards potentially transforming how organizations scale their COVID testing programs. Our enterprise-class TestHub platform has a comprehensive set of features, from bulk purchasing to one-click distribution and easy monitoring of test results, and it stands ready if the FDA authorizes the device.”
Lead Series A investor and Chairman of the Board, Rudy Karsan, details AudibleHealth Dx’s next steps. “RAIsonance’s journey has been remarkable and submitting the application for the EUA is an important next step. If authorized, the test will revolutionize COVID testing for businesses, organizations, and individuals.”
About RAIsonance, Inc.
RAIsonance, Inc., a family of AI-powered technology solution companies headquartered in the Denver Tech Center, is focused on meeting today’s critical health and safety challenges. Founded in March of 2020, AudibleHealth AI, Inc. is the SaMD division of RAIsonance, specializing in AI/ML diagnostic solutions and platforms. Originally funded by an SBIR grant by the National Science Foundation, the team of medical, artificial intelligence, technology, and medical device specialists focuses on developing leading-edge AI/ML-based, scalable, cost-effective diagnostic products across a range of acute and chronic health conditions.
For more information visit us at RAIsonance AI and AudibleHealth AI
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance Releases Preliminary Results from AudibleHealth Dx Validation Study
Updated as of September 6, 2022
Greenwood Village, CO, August 26, 2022 – RAIsonance, Inc., a group of AI-powered technology solutions companies, today reported the preliminary results of its AudibleHealth Dx clinical validation study. The purpose of the trial was to evaluate the AudibleHealth Dx Software as a Medical Device (SaMD) using Forced Cough Vocalization-Signal Data Signature (FCV-SDS) in the diagnosis of COVID-19 illness.
Clinical Study Results
In Q2 of 2022, RAIsonance commenced its Clinical Trial in Florida to validate the AudibleHealth Dx device’s efficacy. Enrollment was 514 participants, with a prevalence of 12.8%. The results of this study will be used to support an Emergency Use Authorization (EUA) submission to the U.S. Food and Drug Administration in the coming weeks.
The study was a prospective, multi-site, non-inferiority trial that compared the AudibleHealth Dx COVID-19 test to a highly sensitive de novo-authorized RT-PCR COVID-19 test when using the AudibleHealth Dx SaMD to detect COVID-19 infections.
For the clinical validation study, the AudibleHealth Dx SaMD’s ability to correctly diagnosis COVID-19 was compared to the BioFire RP2.1 Panel (the first FDA de novo-authorized test for COVID-19). The BioFire RP2.1 Panel runs on the BioFire® FilmArray® Torch and the BioFire® FilmArray® 2.0 Systems in laboratories certified to perform CLIA high complexity or moderate complexity tests.
When compared to this highly sensitive reverse transcription-polymerase chain reaction (RT-PCR) test, the AudibleHealth Dx has results, updated as of September 6, 2022, demonstrating Positive Percent Agreement (PPA) of 84% and a Negative Percent Agreement (NPA) of 85%.
Study participants included males and females aged 18 and older who presented for elective, outpatient COVID-19 RT-PCR testing and met the indications for use for the RT-PCR nasal swab test for COVID-19 using the comparator test and AudibleHealth Dx. All participants stated their willingness to comply with all trial procedures, and informed consent was obtained prior to testing.
The validation study included symptomatic and asymptomatic COVID-19 patients as well as healthy subjects who each utilized the AudibleHealth Dx SaMD device on a mobile phone and then immediately were swabbed with an RT-PCR test.
Usability Analysis Results
In addition to evaluating the NPA and PPA of the device, the company also conducted a separate, comprehensive Usability Analysis. Total net usability enrollment was 443 participants. Demographics included those between the ages of 18 and 88 as well as notable diversity in both ethnicity/race and educational level. Key Usability Analysis outcomes:
- 97% of participants completed the screens of the application completely independently.
- Over 90% felt confident they would know how to receive their results at home.
- 91% felt they would know how to handle a positive or a negative result correctly.
- 97.7% responded that they had a very easy or easy overall experience using the app.
- 98.4% responded that the app screens were very easy or easy to understand.
- 97.5% stated they would be very likely or likely to choose to take an AudibleHealth Dx test at home over other testing options.
- Average time to complete the test, from beginning the registration steps to the delivery of results, was 5.39 minutes.
- Overall successful test completion rate was 99.39%
Background
The use of signal data signatures is common practice for biometric authentication and other means but has not yet been used within the medical community. A forced cough vocalization (FCV), the sound produced when a person intentionally coughs, is unique to each individual and also takes on unique qualities if the individual has certain illnesses, such as COVID-19. RAIsonance has taken this knowledge and the field of artificial intelligence and machine learning (AI/ML) to design and develop a SaMD that uses these unique signatures to diagnose COVID-19 illness.
A Complete Solution
The device, called the AudibleHealth Dx, is designed to diagnose COVID-19 by analyzing the sound of a person’s forced cough vocalization using artificial intelligence. The user interface takes the form of a mobile app. Once the user downloads and registers the app, they cough 4 to 6 times into their mobile phone, and in about 2 minutes, after submitting a cough, they receive their test result in the app. The (SaMD) approach allows for fast and easy COVID-19 testing with no swabs, no lines, no waste, no expiration dates, and no waiting.
The device is designed so that results are also pushed to a comprehensive Patient Portal where users can learn more about the test, the science, and current public health recommendations regarding COVID-19. Thanks to a technical integration with the Association of Public Health Laboratories (www.APHL.org), the platform is also designed to automatically send any positive results to local public health authorities in all 50 states and 4 U.S. territories.
In addition to the mobile application, the company has also designed a web-based, enterprise class, fully integrated testing solution platform called TestHub, designed for high-volume purchasers. Businesses, employers, universities, sports teams, organizations, and government agencies will be able to use TestHub to acquire any quantity of tests, if authorized by the Food and Drug Administration (FDA), and distribute those tests in any quantities to their users, either once or on a recurring basis, with just a few clicks.
The TestHub dashboard is also designed to enable these high-volume users to monitor test usage and results to manage risks and support whatever testing protocols they may have in place. In response to the evolving nature of the COVID-19 pandemic, this seamless platform is nimble and versatile enough to flex with policy changes and infection rates.
Of the results, RAIsonance Founder and CEO, Kitty Kolding, said, “We are incredibly proud of the results of these studies. We spent two years refining this technology and building the device itself and were met with dozens of significant challenges along the way. And to ensure that this test can meet the complex testing needs of organizations, we also built a fully integrated, easy to use ecosystem to ensure that users– individuals, businesses, medical professionals, universities, and government agencies – can experience the full benefits of a totally digital COVID-19 testing solution. The combination of our convenient, mobile-app testing interface and our TestHub portal for high-volume purchasers, we believe, is nothing short of revolutionary.”
“We are delighted at the results of this study, which was the culmination of two years of intense work and intentional design. As a physician, the utility of a testing solution like this is a dramatic step forward in terms of scale, innovation, ease of use, convenience, and efficiency. We believe it will be an impressive and impactful tool for mitigating the spread of COVID-19, if authorized by FDA,” added Karl Kelley, MD, FAADM, RAIsonance’s Chief Medical Officer.
Mona Kelley, Chief of Clinical and Regulatory Affairs for RAIsonance is also pleased with the results. “Given the novelty of the test, understanding the usability of the test was essential. We are very excited about our Usability Analysis results and how well the device performed in that regard.”
“I am so proud of the SaMD that we’ve built. Not only is it a complete solution, but it’s also almost infinitely scalable, cost-effective, and user-friendly. Seeing these study results is incredibly gratifying as the validation for all of our work over the past two years,” said Mark Fogarty, Chief Technical Officer (CTO) and Chief Biotechnical Engineer (CBTE).
Lead Series A investor and Chairman of the Board, Rudy Karsan, details AudibleHealth Dx’s next steps. “Our SaMD has the potential to add a powerful new tool to conduct COVID-19 testing. With this novel technology, we can facilitate mass testing without supply-chain headaches, inventory issues and excessive costs. We expect this integrated platform, if authorized by FDA, to become indispensable for businesses, healthcare providers, universities, government bodies and any other type of organization that would benefit from routine COVID-19 testing. The scalability is virtually limitless.”
About RAIsonance, Inc.
RAIsonance, Inc., a family of AI-powered technology solution companies headquartered in the Denver Tech Center, is focused on meeting today’s critical health and safety challenges. Founded in March of 2020, AudibleHealth AI, Inc. is the SaMD division of RAIsonance, specializing in AI/ML diagnostic solutions and platforms. Originally funded by an SBIR grant by the National Science Foundation, the team of medical, artificial intelligence, technology, and medical device specialists focuses on developing leading-edge AI/ML-based, scalable, cost-effective diagnostic products across a range of acute and chronic health conditions.
Learn more about AudibleHealth AI
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance Appoints Rudy Karsan as Chairman of the Board
Denver, CO, June 10, 2022 – RAIsonance, Inc., a group of AI-powered technology solutions companies that is revolutionizing the COVID-19 testing space and helping to meet today’s critical global health challenges, is proud to announce the appointment of Rudy Karsan as its new Chairman of the Board.
“As a company, we are truly thrilled to announce this appointment,” said Kitty Kolding, Founder and CEO of RAIsonance. “It’s especially gratifying for me personally as well, given the powerful advantages Rudy has already brought us as our lead Series A investor and Board Member.”
Mr. Karsan has a long track record as a highly successful entrepreneur and investor who thrives on building companies from the ground up, whether directly or by actively supporting the entrepreneurs he backs. Mr. Karsan led RAIsonance’s Series A investment in March of 2022 to support the company’s anticipated Emergency Use Authorization filing for its groundbreaking new Software as a Medical Device (SaMD) called AudibleHealth Dx. This new software is designed to diagnose COVID-19 in about 2 minutes by conducting an artificial intelligence-powered analysis of the sound of a user’s cough via a mobile application.
“After working closely with him for nearly a year, I realized Rudy would bring a powerful, additional set of capabilities, relationships, and experience to our team in the role of Chairman,” said Kolding. “I’m so pleased that he’s accepted this role. We have significant global health ambitions for this company, and as my partner, Rudy will help us do exactly what we set out to do: change the face of diagnostic testing and meaningfully impact global health.”
Before joining RAIsonance, Mr. Karsan founded and led Kenexa, a leading global provider of business solutions for human resources. The firm completed an IPO in 2005 and was acquired by IBM in December 2012 for $1.3 billion to provide a platform for its Smarter Workforce initiative.
Since 2014, Mr. Karsan has served as managing director of Karlani Capital, a fund devoted to one main objective: enabling the growth and success of ideas that are original, interesting, and useful to people. Karlani Capital wields the expertise and experience of developing companies from one employee to a 1,000 staff and of growing companies from a single dollar to more than a $1 billion in valuation. The firm has assisted entrepreneurs taking their company into the public markets. This is yet another reason why RAIsonance is excited to have Mr. Karsan on board as the company sets its sights on building out its global health operations.
“RAIsonance and Kitty captured my imagination from the word ‘cough’” said Karsan. “This novel technology is designed to allow you to carry a COVID-19 test in your pocket wherever you go, cutting costs and bypassing or solving supply chain challenges, with the goal of revolutionizing COVID testing as we’ve come to know it over the last several years. I am very blessed and fortunate that Kitty honored me with this position. I look forward to serving to the best of my ability and will work diligently to help bring forth her vision to meaningfully impact global health.”
About RAIsonance, Inc.
RAIsonance, Inc., a family of AI-powered technology solution companies headquartered in the Denver Tech Center, is focused on meeting today’s critical health and safety challenges. Founded in March of 2020, AudibleHealth AI, Inc. is the Software as a Medical Device (SaMD) division of RAIsonance specializing in AI/ML diagnostic solutions and platforms. Originally funded by an SBIR grant by the National Science Foundation, the team of medical, artificial intelligence, technology, and medical device specialists focus on developing leading-edge AI/ML-based, scalable, cost-effective diagnostic products across a range of acute and chronic health conditions.
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance Empowers Businesses and Consumers with SoundPass Technology
Multi-Factor Authentication with Signal Data Signatures is a perimeter security game-changer
Denver, CO, August 5, 2021 – RAIsonance, Inc., a family of Artificial Intelligence/Machine Learning (AI/ML)-based technology companies headquartered in the Denver Tech Center, announced the launch of one of its newest and most innovative products, SoundPass.
SoundPass is a non-medical, biometric tool using multi-factor authentication (MFA) for establishing and maintaining a bubble of perimeter security around the premises or a group of people.
Captured by the mobile app and sent to the AI/ML algorithms, a forced cough vocalization creates a signal data signature or SDS. Our proprietary AI/ML system compares the incoming SDS to the user's personal Baseline SDS for multi-factor authentication. Within minutes, if the new SDS matches the Baseline, the user receives an “Authenticated” message. SoundPass Plus users also receive a QR code for verification at locations using the SoundPass Access software.
"This simple-to-use phone app makes it easy for anyone to conduct a self-check at any time.” said Kitty Kolding, CEO of RAIsonance, Inc. "Our goal is to allow everyone to create a bubble around themselves, their loved ones, friends, even their customers and employees based on individual biometrics. No questionnaires, no medical screenings, no lab testing, no chemical sniffers, no waiting. This is exactly what SoundPass technology delivers.”
Via Apple App Store and Google Play store, SoundPass is available now to businesses, educational campuses, entertainment venues, and any facility seeking to enable smarter in-person interactions.
For more information on SoundPass please visit SoundPass.ai/.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the biometrics, security, and healthcare diagnostics markets. Funded in part with a grant from the National Science Foundation, RAIsonance has developed technology innovations that use Signal Data Signature recognition as a biomarker for non-medical software and a new generation of diagnostic software as medical devices.
For more information on RAIsonance, please visit RAIsonance.ai/.
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance Launches SoundPass Biometric Security Tool
SoundPass uses artificial intelligence to create a biometric perimeter security bubble around workplaces, entertainment and consumer venues, and college campuses.
By Nathan Eddy | August 16, 2021 | mobihealth news
RAIsonance, a group of AI-powered companies focused on natural language processing and AI/ML, announced the release of SoundPass, a trio of AI apps that transform sound data signatures into biometric signal data signatures.
SoundPass uses artificial intelligence to create a biometric perimeter security bubble around workplaces, entertainment venues, public-facing businesses, and college campuses.
The product was designed to bring students back to school and employees back to the office this fall with scalable, efficient controls in place that address the challenges society is currently encountering.
The app learns the unique forced cough signature of each user, which is then securely stored as that user’s baseline for future comparison. If the signature is the same, the user gets an “authenticated” message.
However, if the signatures do not match, the user gets a “not authenticated” message, providing an early warning of a possible problem. Conditions that can alter a baseline forced cough sound include illness and environmental exposure.
The apps come in three editions, Personal, Plus and Access. Personal is free of charge. Plus costs $25 per person, per year, and generates a scannable QR code each time a user authenticates a new forced cough signature to their baseline. The Access app is used to scan the QR code of anyone using SoundPass Plus.
Founded in 2020 in response to COVID-19, and partially funded by National Science Foundation, RAIsonance’s solutions target safety, security and healthcare diagnostics markets, using signal-data signature recognition as a biomarker for human medical diagnostics and in support of safety applications.
"We see a significant opportunity going forward for many different players, some of which we’re actively working to partner with,” Kitty Kolding, CEO of RAIsonance, told MobiHealthNews. “For ourselves, given the approach we take to using AI/ML for security and health purposes, the opportunities are wide open.”
She noted RAIsonance has at least two new products in development to be released on their 2021 road map, all using the same basic capabilities they’ve developed, but serving specific, timely needs in the U.S. and the world.
RAIsonance also has a second product under development, an AI software-as-a-medical device (SaMD) diagnostic for a range of respiratory conditions.
The company recently joined Microsoft for Startups program, giving RAIsonance access to the tech giant’s technologies, including Azure, and to a streamlined path to selling alongside Microsoft and its global partner ecosystem.
“The Microsoft for Start Ups program is really incredible for a start-up,” Kolding said. “Part of the value is the series of credits they offer for software and Azure server utilization which is direct savings for us, and has allowed us to expedite our extensive AI/ML testing with very high capacity CPU and GPU servers.”
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
Former Neustar Executive Joins Denver AI company
Paul McLenaghan Joins RAIsonance as Head of Market Development
Denver, CO, July 23, 2021 – RAIsonance, Inc., a group of AI powered technology solutions companies which help to meet today’s critical health and safety challenges, is proud to announce Paul McLenaghan to its team as Senior Vice President, Market Development.
Before joining RAIsonance, McLenaghan spent 20 years in the advertising technology industry, most recently at Prohaska Consulting, where he worked with major ad tech platforms, publishers, and media companies on data and new product strategy. A market and business development executive with a passion for driving market disruption and transformation, McLenaghan is a versatile leader who combines an ability to generate revenue growth through interaction with external stakeholders, the ability to influence internal product innovation, and the know-how in developing go-to market strategies for new products and technology solutions.
In this role with RAIsonance, McLenaghan will be responsible for influencing adoption and utilization of RAIsonance solutions through partnerships with commercial and non-commercial organizations, as well as identifying new market opportunities for the company.
"We are thrilled to have Paul join our team," said Kitty Kolding, CEO of RAIsonance, Inc. "His background and experience with market development will help expose our company and our offerings to the key partners and markets we’re focused on as we roll out our AI/ML technology solutions."
McLenaghan also spent 15 years of his career at companies such as TARGUSinfo and Neustar where he had a track record of success creating new solutions and lines of business at the intersection of technology, data, and media.
He completed his MBA at the University of Maryland Robert H. Smith School of Business and has BA degrees in Economics and English from Villanova University. He is a dual citizen of Australia and the United States.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets. Originally funded with a grant from the National Science Foundation, RAIsonance has developed breakthrough technology innovations that use Signal Data Signature Recognition as a biomarker for human medical diagnostics and to support ground-breaking safety applications. To learn more about SoundPass go to SoundPass.ai/.
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance, Inc. Joins Microsoft for Startups Program
RAIsonance Selected for Microsoft for Startups Program Designed to Help the World’s Most Promising B2B Startups Quickly Scale
Denver, CO, July 1, 2021 – RAIsonance, Inc., a family of Artificial Intelligence/Machine Learning (AI/ML)-based technology companies headquartered in the Denver Tech Center, dedicated to solving today’s critical health and safety challenges, announced today that they have been named the newest member of Microsoft for Startups. This global program provides the tools and resources B2B startups need to rapidly build their business. As a member in the program, RAIsonance will have exclusive access to Microsoft's technologies, including Azure, as well as a streamlined path to selling alongside Microsoft and its global partner ecosystem.
Founded in 2020 in response to COVID-19, and partially funded by National Science Foundation, RAIsonance is a family of companies dedicated to Artificial Intelligence/Machine Learning (AI/ML) based solutions for the safety, security, and healthcare diagnostics markets.
One of the company’s newest products, SoundPass, is a trio of artificial intelligence applications that transforms sound data signatures into biometric signal data signatures.
Users establish a baseline by producing a “forced cough vocalization” multiple times into the app, teaching it to recognize their unique baseline signature. Once this baseline is established, SoundPass users can conduct a self-check at any time to verify whether a change has taken place compared to their own unique baseline.
If nothing has changed, Sound-Pass generates an “authentication” message in under 2 minutes. Users of SoundPass Plus also receive a QR code that can be read by SoundPass Access software at businesses, places of worship, entertainment venues or educational campuses using the SoundPass technology to enhance their safety protocols.
If a user attempts a self-check with SoundPass and is unable to obtain an authentication, this indicates a potential risk. The most common reason for an authentication failure is that the user experienced a change in their cough signature. Conditions that can create such a change to a forced cough sound include illness and environmental exposure, among others.
With support from the Microsoft for Startups program, SoundPass will be able to roll out the SoundPass Access technology rapidly to businesses, educational campuses, entertainment venues, houses of worship or any facility seeking to enable smarter in-person interactions. This rigorous, scientific approach helps employees, customers, visitors and congregants feel more confident and comfortable when returning to in-person interactions and activities.
"We are thrilled to become a part of the Microsoft for Startups program," said Kitty Kolding, CEO of RAIsonance, Inc. "This program will provide us with exposure to thousands of businesses who will utilize our technology solutions to work toward a new normal. Our entire platform has been Azure-based from the start, so we are incredibly excited to collaborate with, and learn from the experts at Microsoft to help build our business faster and better."
"Now is the time to provide peace of mind about returning to in-person activities, and Microsoft is looking forward to working with RAIsonance to make that happen in a bigger way," said Sally Frank, WW Lead, Health & Life Sciences, Microsoft for Startups.
For more information on SoundPass, please visit SoundPass.ai/. For more information on the Microsoft for Startups Program, please visit Microsoft for Startups.
About RAIsonance, Inc.
RAIsonance is a family of companies headquartered in the Denver Tech Center in Colorado, dedicated to Artificial Intelligence/Machine Learning (AI/ML)-based solutions for the safety, security, and healthcare diagnostics markets. Originally funded with a grant from the National Science Foundation, RAIsonance has developed breakthrough technology innovations that use Signal Data Signature Recognition as a biomarker for human medical diagnostics and to support ground-breaking safety applications. To learn more about SoundPass go to SoundPass.ai/.
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai
RAIsonance, Inc. Announces First Medical Advisory Board
AI Technology Company Brings Together Revered Team of Healthcare Professionals from Around the Country
Denver, CO, July 1, 2021 – RAIsonance, Inc., a group of AI powered technology solutions companies which help to meet today’s critical health and safety challenges headquartered in the Denver Tech Center, is proud to announce that it has seated its very first Medical Advisory Board to commence its two-year term starting June, 2021.
The distinguished members include:
- Rodolfo Aldir, MD, FACC, FCCP; Medical Advisory Board Chair; Interventional Cardiologist with Orlando Health
- Lewis Marshall, MD, JD, MS, FAADM, FAAEP, FACP, FACHE, FCLM; Advisory Board Member; Chief Medical Officer at NYC Health & Hospitals / Lincoln; Member and Fellow of the American Academy of Disaster Medicine
- Andrea Keener, PhD, LMHC, MCAP, Long COVID Advisory Board Chair
- Jeanne M. LeBlanc, PhD, ABPP, R. Psych; Advisory Board Member; Private Practice - Clinical Neuropsychology and Rehabilitation
- Frederick L. Slone, MD, FAADM; Advisory Board Member; Associate Professor at University of South Florida College of Medicine, Member and Fellow of the American Board of Disaster Medicine
- Ryan Chakra, MD; Advisory Board Member; Senior Medical Director at BD
- Greg Cohen, DO, FACOFP; Advisory Board Member; Lucas County Health Center Medical Clinics in Chariton, Iowa
- Heidi P. Cordi, MD, MPH, MS, EMTP, FACEP, FAADM; Advisory Board Member; Core Faculty for the Division of Emergency Medical Services, Associate Director of the EMS Fellowship Program, and EMS Coordinator for Rensselaer County, Assistant Professor of Medicine at Columbia University Medical Center, Assistant Professor of Clinical Medicine and Associate Medical Director of Emergency Medical Services at New York Presbyterian Hospital, Medical Director of Mohawk Ambulance in Albany; Member and Fellow of the American Academy of Disaster Medicine
- Elizabeth Maxwell-Schmidt, MS, MD, FAAEP, FACEPMD; Advisory Board Member; Emergency Medicine Specialist in Annapolis, MD
“We are incredibly gratified to have attracted such an esteemed team of brilliant medical professionals to serve on our Medical Advisory Board,” said RAIsonance’s Chief Medical Officer and Chief Strategy & Planning Officer Maurice Ramirez, DO, Ph.D. “With their guidance and support, we will be extremely well-positioned to bring our groundbreaking AI/ML technologies to market. As we seek to make a meaningful impact on the markets we serve, our Advisory Board will provide us with the essential insights we’ll need to make products that serve practitioners and patients all over the US, and indeed around the world.”
Founded in 2020 in an effort to impact COVID-19, and partly funded by National Science Foundation, RAIsonance is structured as a group of separate companies dedicated to Artificial Intelligence/Machine Learning (AI/ML) based solutions for the safety, security, and healthcare diagnostics markets. “We’re incredibly fortunate to have the opportunity to work with and learn from these expert clinicians,” said Kitty Kolding, CEO of RAIsonance, Inc. “Each individual brings unique perspective and invaluable expertise that will shape our product concepts and specialized technologies as we continue our work in health, wellness, biometrics and safety. It’s hard to overstate how thrilled we are to be working with a team of advisors like this one.”
For more information on RAIsonance, please visit RAIsonance.ai/
Investor Contact:
Email: InvestorRelations@raisonance.ai
Media Contact:
Email: Press@raisonance.ai




